July 12, 2021 – Diamonds are a type of gemstone made of carbon atoms arranged in repetitive tetrahedrons. It’s a molecular configuration that imparts diamonds with beauty and molecular brawn. In August 2015 in northern Canada about 130 miles from the Arctic Circle, a 187.63 carat diamond, called the Foxfire diamond, was accidentally mined.
According to the museum, the Foxfire diamond is the largest known uncut, gem-quality diamond mined in North America. Most large diamonds are mined in South Africa and Western Australia where rare blue and pink diamonds are also found deep in the Earth. Diamonds with distinct color result from the presence of trace elements, such as nitrogen and boron, and/or a change in the configuration of the atoms.
Diamond basics
Diamonds are carbon atoms arranged in tetrahedrons. According to information from the Gemological Institute of America, (GIA) diamonds are the only gem with just one element – carbon. Formed billions of years ago about 100 miles deep in the Earth under high pressure, diamonds are the hardest material on Earth.
Pure diamonds are colorless but because trace elements can become integrated into the molecular structure of a diamond, diamonds come in a wide variety of colors. Common trace elements found in diamonds include nitrogen, hydrogen, and boron. Traces of nitrogen give diamonds a yellow color, which can either decrease or increase the value of the diamond depending on the degree of the yellow color. Boron in the carbon tetrahedrons gives the diamond a blue color and is highly valued.
The 29.6-carat rough, uncut ‘Blue Moon Diamond’ is held in this photo by Johan Duppenar, CEO of Petra Diamonds. This photo is from the Nov. 12, 2015 Student News Net story (#8031) about the gem’s auction. (Photo: Petra Diamonds)
Diamonds also show color due to slight changes in the tetrahedral structure of the molecule. Pink diamonds are an example of diamonds in which trace elements cannot be found so the color is thought to be due to slight alteration of the tetrahedron lattice.
This is the Argyle Pink Jubilee diamond found in Western Australia in 2011 at the Rio Tinto mine. (Photo: Rio Tinto)
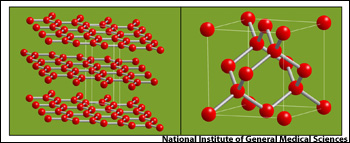
Graphite is also a material made of all carbon atoms but the atoms are arranged differently giving graphite radically different physical properties. Instead of being hard, graphite is so soft, it’s used to make pencils.
On the left is the arrangement of carbon atoms in graphite (planar lattice) and to the right, carbon atoms are arranged in tetrahedrons to form diamonds. (Graphic: National Institute of General Medical Sciences/NIH)
Foxfire diamond
The Foxfire diamond was mined accidentally at the Diavik Diamond Mine in the Barren Lands of Canada’s Northwest Territories near the Arctic Circle. The area has never been known to contain large diamonds. Diamonds found there over the previous decade generally peaked at six carats. Because of this, the mine’s equipment was configured to sift out stones smaller than six carats while pulverizing the larger ones. The 187.63 carat Foxfire diamond should have been crushed, but its uncommonly flattened shape enabled it to safely pass through the filters, according to the museum.
After it was unearthed in August 2015, the large diamond was sold at auction in June 2016 to Deepak Sheth of Amadena Investments LLC. He decided to keep the diamond intact and loan it to the Smithsonian museum where it will be displayed in the Harry Winston Gallery next to the famous Hope diamond, a beautiful blue diamond made into a necklace.
“Having North America’s largest known uncut, gem-quality diamond on display at the Smithsonian is a testament to the rarity of the Foxfire diamond,” Sheth said in a museum press release. “It also represents another significant chapter in the diamond’s remarkable story.”
The Foxfire diamond was named after the aboriginal description of the resplendent northern lights that light up the Arctic sky like a brush of undulating fox tails, according to a museum press release.
Opened in 1910, the museum, one of the most popular natural history museums in the world, has an extensive gem collection where visitors can spend hours viewing gems and learning about gemology.
“The Foxfire is truly exceptional, one of the great treasures of the Earth,” Jeffrey Post, curator of the National Gem and Mineral Collection, said in a press release. “We are delighted that our visitors will have this once-in-a-lifetime opportunity to view North America’s largest gem-quality diamond in its natural form.”
For information on careers in Gemology, visit the Gemological Institute of America (GIA).
Oct. 12, 2020 – More than 90 percent of the world’s pink diamonds come from The Argyle Diamond Mine in Western Australia. It is owned by the Rio Tinto Company. Formed deep within the Earth from one to three billion years ago, pink diamonds are rare (less than 0.03 percent of world production) and very expensive.
All diamonds, regardless of color, are made of carbon (C) atoms, a chemical element.
Diamonds are carbon atoms arranged as tetrahedrons in a lattice. When carbon atoms are configured in parallel layers, the result is not a shiny, spectacular diamond but a black piece of graphite. Grahite is a common material used to make a wide variety of products including the tips of pencils. Even though many people refer to pencils as ‘lead pencils,’ the tips are graphite, not lead. Lead is also a chemical element (Pb) but it is different from carbon (C).
Rocks and even meteorites can contain graphite.
are a tetrahedral lattice
(Graphic: the National Institute of General Medical Sciences/NIH)
Diamonds formed deep in the ground (about 100 miles below the Earth’s crust) under intense pressure (one million pounds per square inch) from one to three billion years ago. From 300 to 400 million years ago, diamonds rose from the depths of the Earth through volcanic eruptions and other geological conditions. Diamonds are unearthed through a mining process. Naturally occurring diamonds are unearthed, not made.
Synthetic diamonds are artificial diamonds that can be made in a lab. Synthetic diamonds are not as valuable as naturally occurring diamonds.
Trace Elements in Diamonds
When there are trace elements in a diamond in addition to the carbon atoms, the gem will take on color. Nitrogen is a common element found in diamonds. Diamonds with nitrogen look yellow because blue wavelengths of light are absorbed. Diamonds are graded, in part, on how yellow the diamond looks.
Blue diamonds contain the element, boron, resulting in the absorption of red, orange or yellow wavelengths of light so the diamond appears blue.
Not all colored diamonds are due to the presence of other elements.
Pink Diamonds
Why some diamonds are pink is still being studied. Evidence does not suggest it is due to the presence of a trace elements. Trace elements have not been found by gemologists and chemists who have studied the unusual gem. The pink color is believed to be due to a shift in the tetrahedron lattice.

in the tetrahedral molecular structure.
(Photo: Rio Tinto)
In August 2011, the Rio Tinto Argyle Diamond Mine in Western Australia found a 12.76 carat pink diamond, the largest one ever found there. As a unique diamond, it was named the Argyle Pink Jubilee. After unearthed, rough diamonds are cut and polished by diamond cutting experts. When experts began examining the Argyle Pink Jubilee diamond, it was only capable of being partially cut and polished. The result was a 8.01 carat pink diamond. Rio Tinto decided to donate it to the Melbourne Museum. It is now on display there.
“As the largest pink diamond found in Australia, this is an important and spectacular piece of Australia’s mining history. We are very appreciative of Rio Tinto for donating the diamond, and are excited to be able to share its story at Melbourne Museum,” Mr. Tim Hart, acting CEO, Museum Victoria, said in a July 2012 press release announcing the donation.
The Rio Tinto mine in Western Australia opened in 1983 and will be closing at the end of 2020 as the yield of diamonds has declined.
Careers
For students interested in learning more about careers in the gem and jewelry industries, the Gemological Institute of America, GIA, has a wide range of diploma programs and courses. (See link #1 below)
REVIEW QUESTIONS
1. If carbon atoms are arranged in layers or planes, what is the substance called?
2. If carbon atoms are arranged in tetrahedrons, what is the substance called?
3. Where do diamonds form? When did diamonds form?
4. Why are some diamonds pink?
5. Why are some diamonds blue?
INQUIRY QUESTIONS
1. Why are pink and blue diamonds more expensive than white diamonds?
2. Why do you think the Rio Tinto Mine in Western Australia had to close?

